How Many Electrons Are in a P Sublevel
The p sublevel has 3 orbitals so can contain 6 electrons max. The s-sublevel is made up of a singular orbital holding a maximum of 2 electronsIt is a spherical shape.
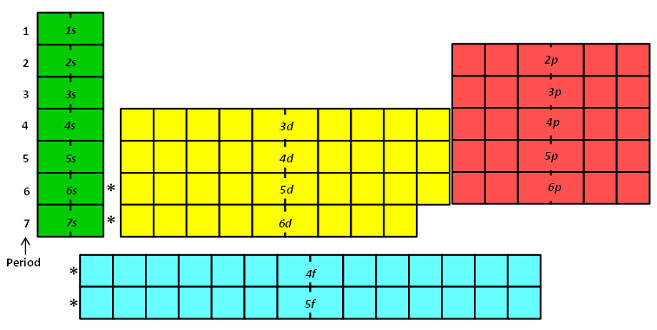
Electron Configuration Texas Gateway
The d sublevel has five orbitals and can contain 10 electrons.

. Level 1 does not have a p or d or f sublevel only an s sublevel. The p-sublevel is made up of a 3 identical dumbbell like orbitals. If we add the number of electrons that each sublevel holds it looks like this.
36 How Many orbitals are there in n3 the 3rd energy level of an atom. The number of orbitals in a shell is thesquare of the principal quantum number. How many orbitals in the p sublevel.
Some things to notice. The d sublevel has 5 orbitals so can contain 10 electrons max. 35 S P D F orbitals Explained 4 Quantum Numbers Electron Configuration.
And the 4 sublevel has 7 orbitals so can contain 14 electrons max. There is one orbitalin an s subshell l 0 three orbitals in a p subshell l 1 and five orbitals in a d subshell l 2. What can n equal.
In the picture below the orbitals are represented by the boxes. How many orbitals in the d sublevel. So in order to have a p-sublevel you need to have n1 since the first energy level.
33 How many electrons can occupy a 6f orbital. The diagram below really shows the overlap of the Principal Energy Levels. Sublevel l 3 indicates an f sub shell.
32 What is the L value of the p sublevel. The s subshell which has 1 orbital with 2 electrons the p subshell which has 3 orbitals with 6 electrons the d subshell which has 5 orbitals with 10 electrons and the f subshell which has 7 orbitals with 14. The p sublevel has three orbitals and can contain 6 electrons.
34 How many electrons are in a 4d sublevel. And the 4 sublevel has 7. Also no two electrons in an atom can have the same four quantum numbers as stated by the Pauli Exclusive Principle.
How many orbitals in the f sublevel. The numberof orbitals in a subshell is therefore 2l 1. It is any positive integer value so cannot be 0 or -1.
The p sublevel has 3 orbitals so can contain 6 electrons max. 12 122 4 32 9. You can put two electrons in each box.
Thus the f subshell has seven orbitals. The 1s is the closest to the nucleus and is smaller that the 2s which is smaller than the 3s and so on. The d sublevel has 5 orbitals so can contain 10 electrons max.
The fourth shell has 4 subshells. The f sublevel has. SImply put the principal quantum number gives the energy level on which the electron resides the angular momentum quantum number gives you the sublevel and the number of values you have for the magnetic quantum number gives you the number of orbitals you get per sublevel.
Within the f sublevel l 3 there are 7 orbitals.

0 Response to "How Many Electrons Are in a P Sublevel"
Post a Comment